

Other arrangements are oxygen with 1 bond and 3 lone pairs, that has a -1 formal charge, and oxygen with 3 bonds and 1 lone pair that has a formal charge of +1. The common arrangement of oxygen that has a formal charge of zero is when the oxygen atom has 2 bonds and 2 lone pairs. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. Because this book concentrates on organic chemistry as applied to living things, however, we will not be seeing ‘naked’ protons and hydrides as such, because they are too reactive to be present in that form in aqueous solution. The hydrogen radical is a hydrogen atom with no bonds, a single unpaired electron and a formal charge of 0. The hydride ion is a is a hydrogen with no bonds, a pair of electrons, and a formal charge of -1. The proton is a hydrogen with no bonds and no lone pairs and a formal charge of +1. The exceptions to this rule are the proton, H +, the hydride ion, H -, and the hydrogen radical, H. The common bonding pattern for hydrogen is easy: hydrogen atoms in organic molecules typically have only one bond, no unpaired electrons and a formal charge of zero. More importantly, you will need, before you progress much further in your study of organic chemistry, to simply recognize these patterns (and the patterns described below for other atoms) and be able to identify carbons that bear positive and negative formal charges by a quick inspection. You should certainly use the methods you have learned to check that these formal charges are correct for the examples given above. You may encounter carbenes in more advanced chemistry courses, but they will not be discussed any further in this book. Carbenes are a highly reactive species, in which a carbon atom has two bonds and one lone pair of electrons, giving it a formal charge of zero. Carbon radicals have 7 valence electrons and a formal charge of zero.
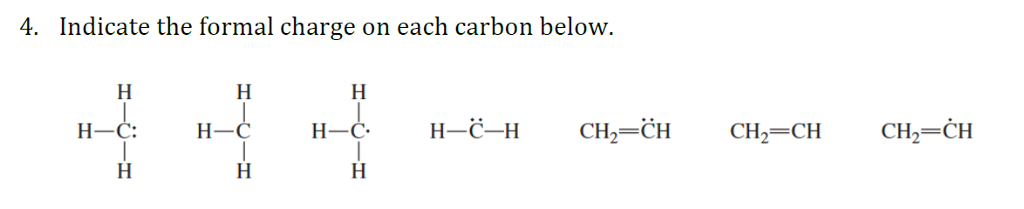
A carbon radical has three bonds and a single, unpaired electron. Two other possibilities are carbpon radicals and carbenes, both of which have a formal charge of zero. Carbanions have 8 valence electrons and a formal charge of -1.
Formal charge of carbon plus#
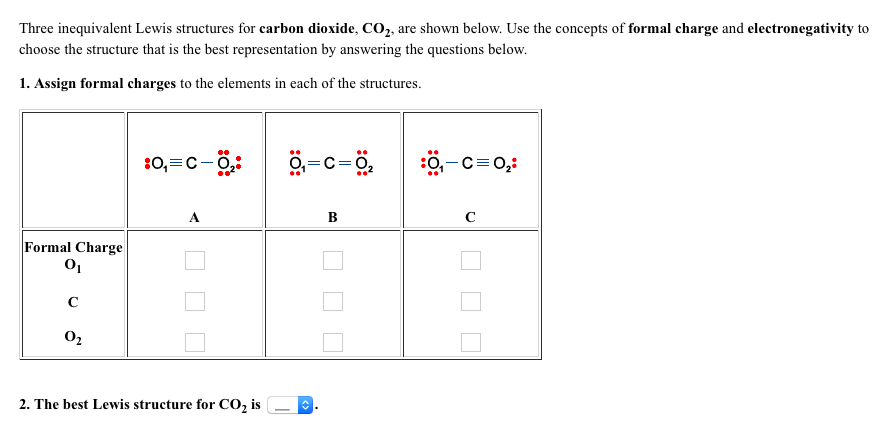
Carbanions occur when the carbon atom has three bonds plus one lone pair of electrons. Carbocations have only 6 valence electrons and a formal charge of +1. Carbocations occur when a carbon has only three bonds and no lone pairs of electrons. Later in this chapter and throughout this book are examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom has a positive or negative formal charge, respectively. In other words, carbon is tetravalent, meaning that it commonly forms four bonds.Ĭarbon is tetravalent in most organic molecules, but there are exceptions. And each carbon atom has a formal charge of zero. In the structures of methane, methanol, ethane, ethene, and ethyne, there are four bonds to the carbon atom. The formal charge of each atom in a molecule can be calculated using the following equation:Ĭarbon, the most important element for organic chemists. Bonding electrons are divided equally between the two bonded atoms, so one electron from each bond goes to each atom.Non-bonding electrons are assigned to the atom on which they are located.

To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules: Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. Students will benefit by memorizing the "normal" number of bonds and non-bonding electrons around atoms whose formal charge is equal to zero.Ī formal charge compares the number of electrons around a "neutral atom" (an atom not in a molecule) versus the number of electrons around an atom in a molecule. It is more important that students learn to easily identify atoms that have formal charges of zero, than it is to actually calculate the formal charge of every atom in an organic compound.


 0 kommentar(er)
0 kommentar(er)
